Conventional batteries are a lot like camels. They’re great for storage and transportation, but they’re not exactly speedy.
For technologies that require a fast discharge of energy, such as heart defibrillators, alternative materials are often used, foremost among them, antiferroelectrics.
There are only a handful of known antiferroelectric materials, and most of them contain lead, so they aren’t safe enough for everyday applications. Now, a Cornell-led collaboration has discovered a new approach for making a lead-free antiferroelectric that performs as well as its toxic relatives.
The team’s paper, “Liberating a Hidden Antiferroelectric Phase with Interfacial Electrostatic Engineering,” published Feb. 2 in Science Advances. The senior author is Darrell Schlom, the Herbert Fisk Johnson Professor of Industrial Chemistry in the College of Engineering. Julia Mundy, Ph.D. ’14 of Harvard University; Bastien Grosso of ETH Zürich; and Colin A. Heikes, Ph.D. ’15 of the National Institute of Standards and Technology, Center for Neutron Research, are co-first authors.
Like their better known magnetic cousins, ferroelectrics and antiferroelectrics are a class of material that has bits of information, called dipoles, arranged in a pattern. In ferroelectrics, the dipoles are oriented in the same direction. In antiferroelectrics, the dipoles alternate, i.e., up, down, up, down. Since the adjacent dipoles cancel each other out, the material has no net polarization.
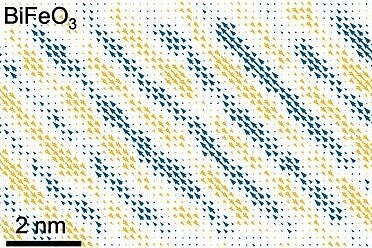
Initially, the researchers set out to experiment on thin films of bismuth ferrite – a ferroelectric with the highest polarization of any known material. They stacked alternating layers of bismuth ferrite with other insulators to create a structure resembling the exquisite torte of a European baker, except that the individual layers of this ferroelectric sandwich are each less than a thousand times thinner than the diameter of a human hair. As the ferroelectric layers got thinner, something quite unexpected happened. The researchers discovered that their ferroelectric with super high polarization was suddenly transmuted into an antiferroelectric with no net polarization. In so doing, a previously unknown ground state that would never have been possible in a bulk crystal was born.
“Normally, we always study the most stable form of a material – its lowest energy state,” said Mundy. “By using our thin film technology, we found that it became possible to stabilize what is normally an inaccessible higher energy state. In this case, that higher energy and previously unknown state has quite useful properties.”
Working with David Muller, the Samuel B. Eckert Professor of Engineering, the team was able to take a deeper look into the material’s “hidden” state with an electron microscope pixel array detector (EMPAD) capable of seeing atoms at record resolution. This allowed the researchers to see the electric fields inside the material and, for the first time, image the polarizations of an antiferroelectric’s dipoles.
The behavior of these dipoles is essentially a “battle of two giants,” according to Schlom, with the ferroelectric material attempting to polarize the neighboring insulating layers in the stack, and the neighboring insulating layers trying to resist being polarized. To understand these electrostatic interactions, and the theory that explains the new antiferroelectric they stabilized, the Cornell team worked with collaborators from Harvard; Pennsylvania State University; University of California, Berkeley; ETH Zürich; Oak Ridge National Laboratory; and the Leibniz Institute for Crystal Growth.
“It’s hard to see metastable things; even the theorists hadn’t realized that bismuth ferrite could assume this form,” Schlom said. “But Julia saw it and, with electron microscopy, she was able to map the polarizations and see that, wow, it looks, smells and feels like an antiferroelectric. With clues of its existence, the theorists were able to precisely describe the structure and stability of this new ground state and establish how electrostatics perturbs the energetic landscape to make this antiferroelectric form the stable state.”
Schlom calls this new ground state a “polymorph” because it still possesses the same atoms, with only a slight structural change.
For two decades, Schlom’s research group has focused on altering the energetic landscapes and properties of materials by applying strain: stretching the thin films on top of a single-crystal substrate. Now, electrostatic engineering has provided a new tool, one that is electrical rather than mechanical.
“What’s beautiful about this material is the theory shows that strain alone cannot stabilize this antiferroelectric state. So this is a new knob,” Schlom said. “And I think there’s a lot of applications for this material where you want to get the lead out. Antiferroelectrics are used in capacitors; it’s already in thin film form. That makes our stacking approach to nullify the need for lead quite applicable.”
Plant Engineering - By David Nutt February 11, 2022
https://www.plantengineering.com/articles/electrostatic-engineering-gets-the-lead-out-for-faster-batteries/